how does a car battery work physics
Most modern cars require relatively low cold cranking amps that range from 400 to 600. P 600 A 72 V P 4320 W.
A battery is a device that stores chemical energy and converts it to electrical energy.
. The flow of electrons provides an electric current that can be used to do work. In 1866 a French engineer Georges Leclanche developed a new kind of battery. The terminals are two raised pieces at each end of the battery that transmit energy from the battery into your car.
The starter has much less resistance than the bad battery so most of the current will go through the starter allowing a jump start to work. So a car has a rechargeable battery and a charging system to keep it topped up. A battery is a device that transforms chemical energy to electric energy and that is the basic way a car battery works.
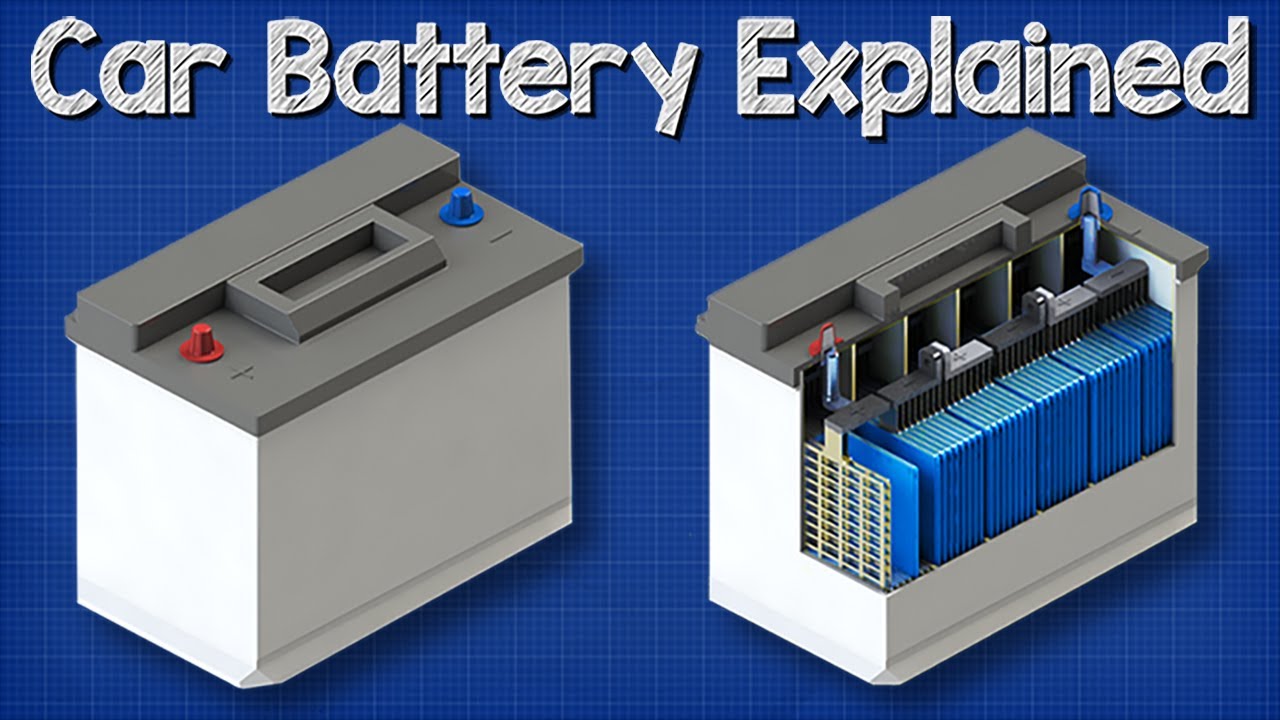
How does a car battery work learn from the basics where we use and battery and how batteries work. A car battery is actually 6 smaller batteries that are lined up in series. A car battery uses lead-acid technology to turn chemical energy into electricity.
Up to 5 cash back In a working battery with no cracks or breaks the only parts of the battery that are visible are the casing and the terminals. This causes the voltages of each battery to add. If an electrical appliance like a light bulb motor or.
With thanks to Squarespace for sponsoring this video. To find the power of a car battery we multiply the CCA number by 72 volts. In most car batteries you have six cells and therefore a 12-volt battery.
The key is the different electrochemical properties of the Zn and Cu ions described on other battery questions on this site. How does a car battery work physics. When we work or run the stored chemical energy in our body is converted into heat or kinetic energy.
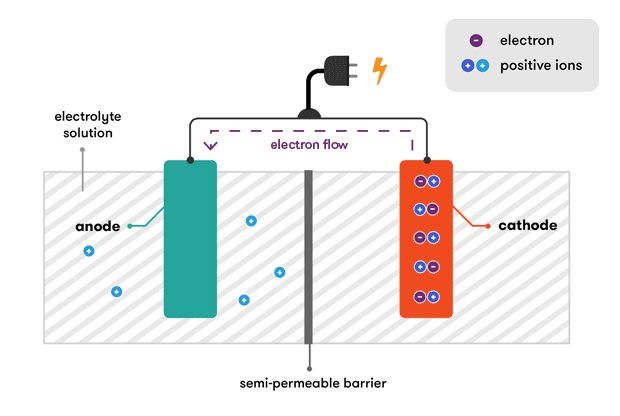
To balance the flow of electrons charged. The lead-acid battery was the first form of rechargeable secondary battery. The chemical reactions in a battery involve the flow of electrons from one material electrode to another through an external circuit.
Batteries are made up of one or more cells each containing a positive electrode negative electrode and an electrolyte. Rechargeable batteries like the kind in your cellphone or in your car are designed so that electrical energy from an outside source the charger that you plug into the wall or the dynamo. It was a carbon-zinc wet cell battery known as the Leclanche cell.
So batteries are just devices that convert chemical energy into electricity. There are 2 connectors that go out of the. The positive terminal is red and the negative terminal is black.
The electrodes in the battery contain atoms of certain conducting materials. The plates are submerged in sulphuric acid that triggers a reaction between the two plates. It is still the most popular to be used as a car battery.
For instance in an alkaline battery. The chemical energy results from the lead-acid reaction that begins the whole process. As the ions move freely around the lead plates another chemical reaction occurs which produces hydrogen and lead sulfate.
Inside the battery are 3 important things. The lead-acid battery is still in use for many industrial purposes. A typical SLI battery has six cells.
Each cell is able to produce about 2-volts of energy. Jump starting a car refers to recharging a dead uncharged car battery just enough to get the starter motor rotating. However as long as there enough power from the battery to run the cars electronic engine controller and ignition system the engine can.
These batteries only work in one direction transforming chemical energy to electrical energy. Each cell has two plates or grids. Sports cars and light trucks require higher cranking amps ranging from 700 to 1000 A.
The movement of ions generates electricity that moves to either the positive or negative terminal of. To kickstart the chemical reactions in the battery you just connect a wire between its negative and positive terminals and a steady stream of electrons a current is produced as the reactions get under way. All of the parts of the battery work together to make the flashlight light up.
If the bad battery is shorted then it will have to be disconnected before a jump start will work. The general way that a battery works is that when an electronic circuit is connected to the battery electrons are allowed to flow. An electrical cell is a device used to produce electricity.
But in other types of batteries the reaction can be reversed. As such the car battery is categorised as a. So the solution properties of the ions not just the Fermi levels of the electrons enters into the battery.
One or more cells are used as the batteries to produce. One is made of lead the other of lead dioxide.

How Do Batteries Work Weego Portable Power

How A Car Battery Works The Engineering Mindset

How A Car Battery Works The Engineering Mindset

How A Car Battery Works The Engineering Mindset
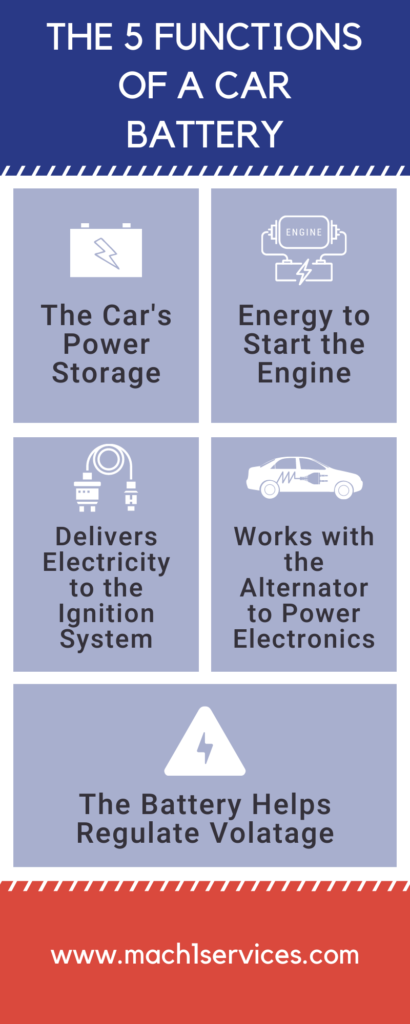
The 5 Functions Of A Car Battery Mach 1 Services
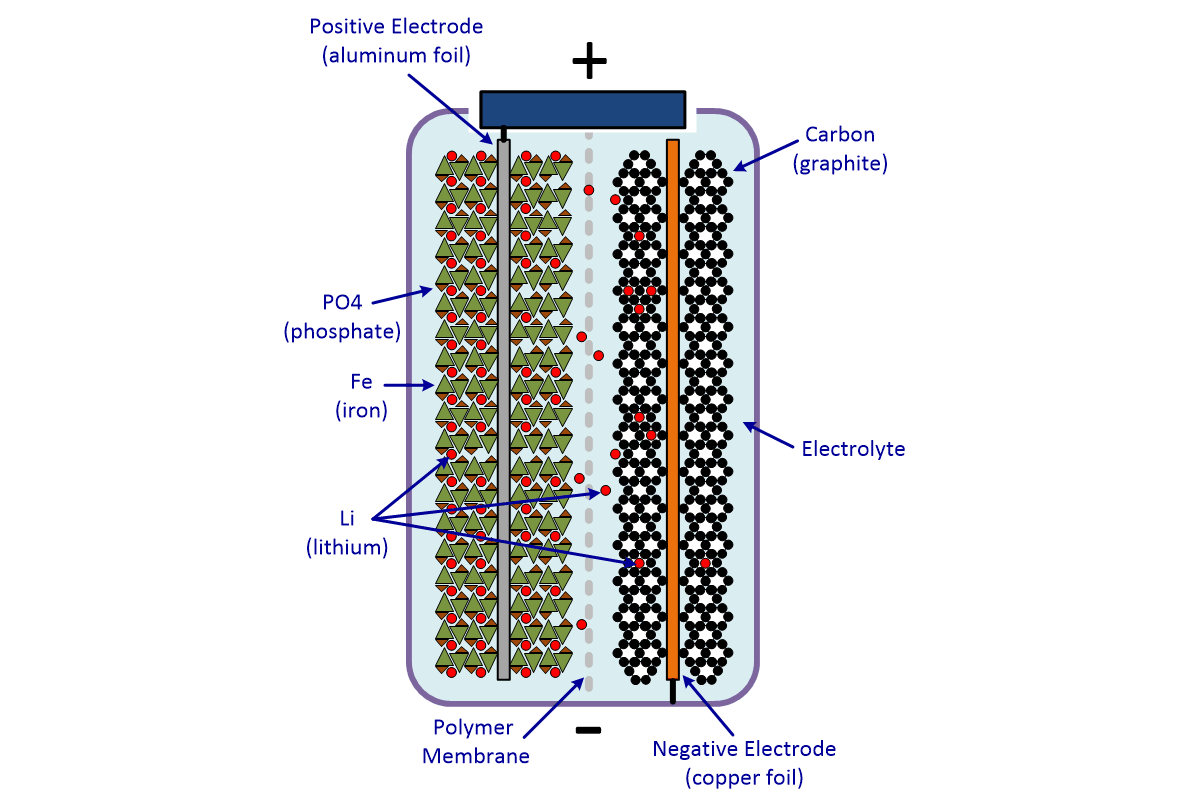
Electric Car Battery Chemistry What S The Difference Carexpert

How A Car Battery Works The Engineering Mindset

Lithium Ion Battery Packs And Cells Come In All Shapes And Sizes But They Re Lithium Ion Batteries Rechargeable Batteries Recondition Batteries

How A Car Battery Works Youtube

How Electric Cars Work Animagraffs

How Does An Electric Bell Work Using Electromagnets
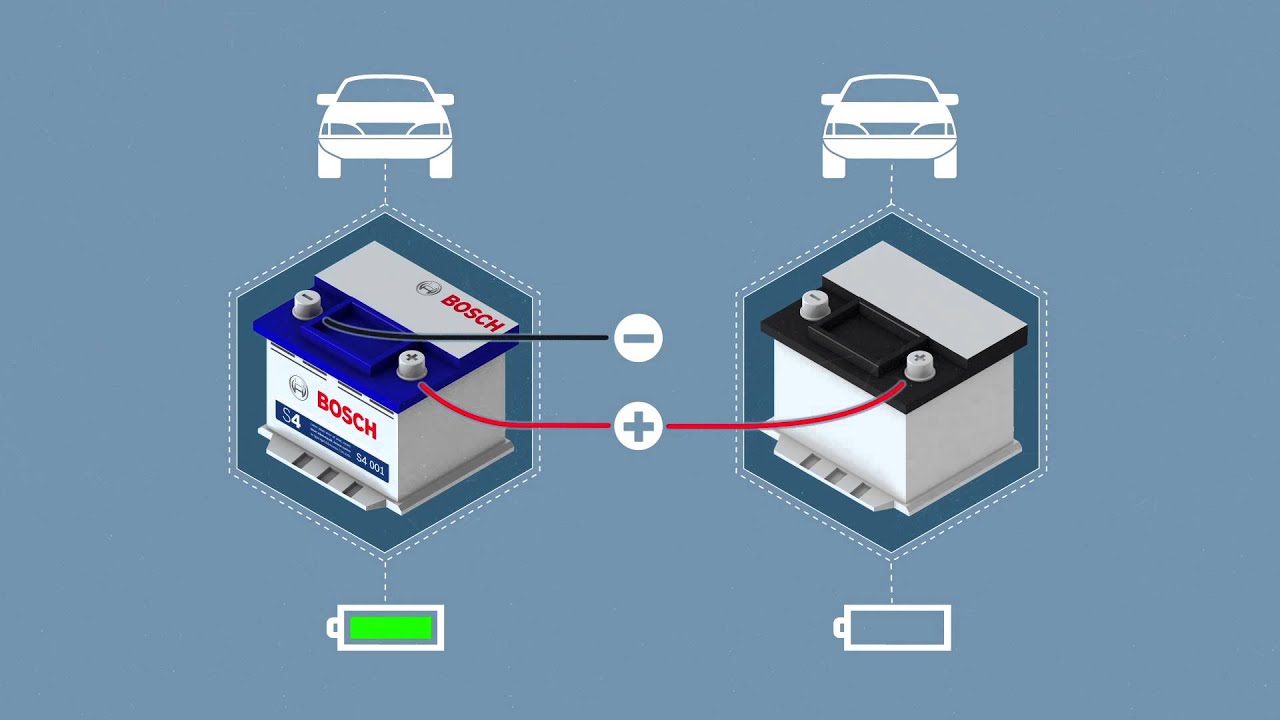
En I Battery Basics How Does A Jump Start Really Work Youtube

Battery Working Principle How Does A Battery Work Electrical4u
How Does A Car Battery Work Mach 1 Services

How A Car Battery Works Basic Working Principle Youtube
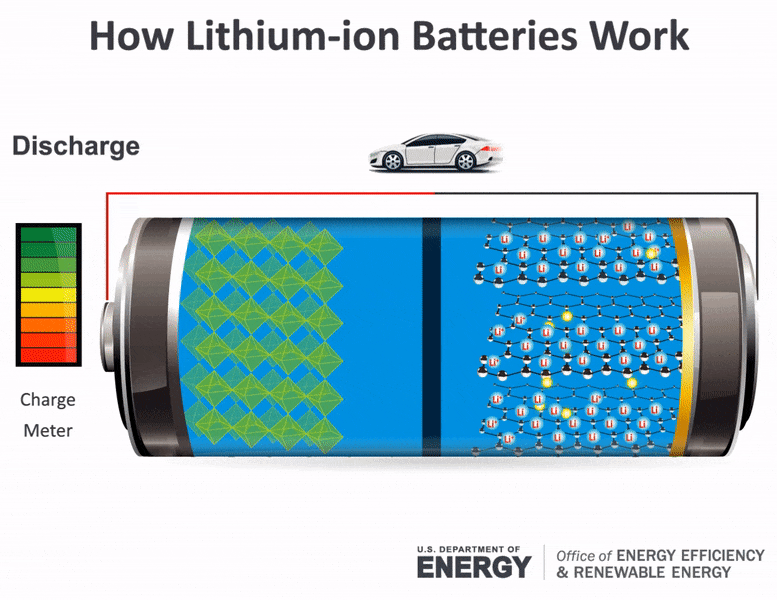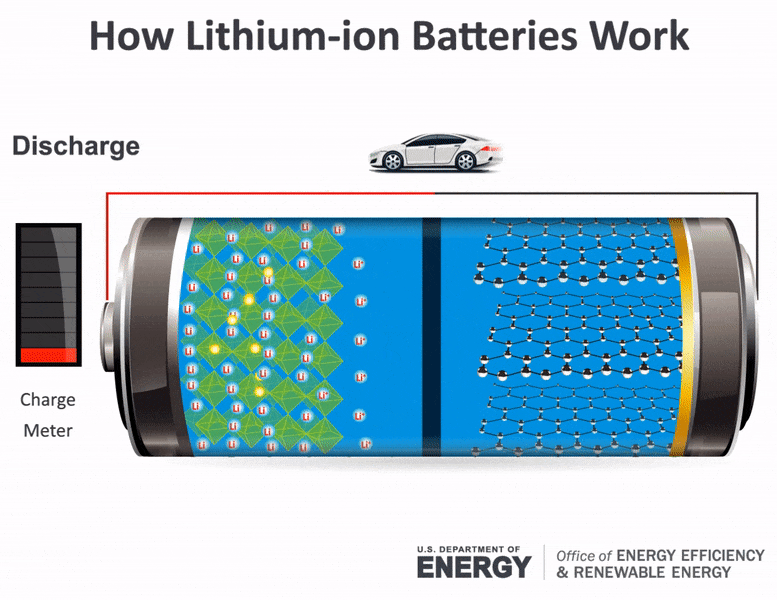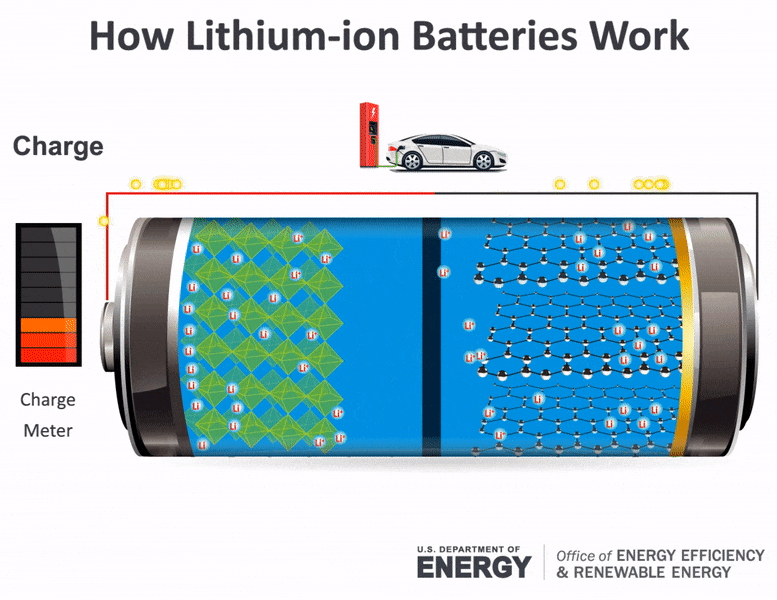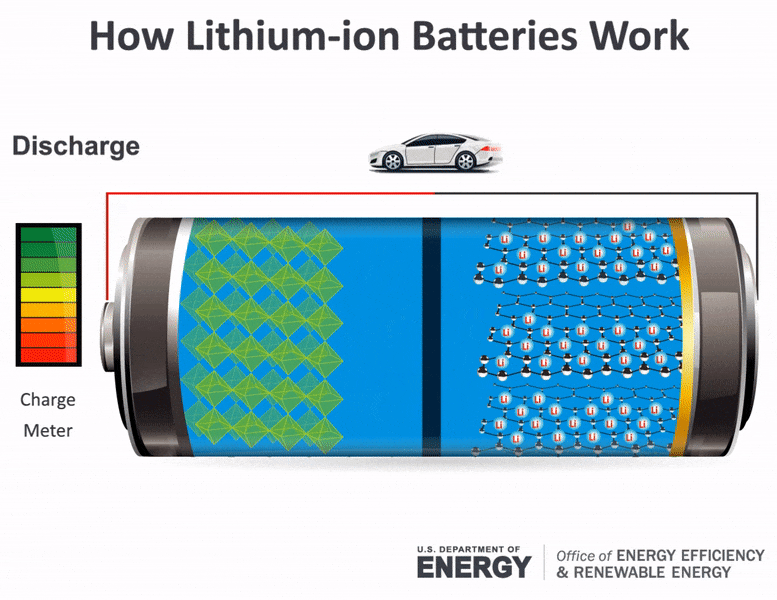
Science Made Simple What Are Batteries And How Do They Work